The Promise of Proven Purity
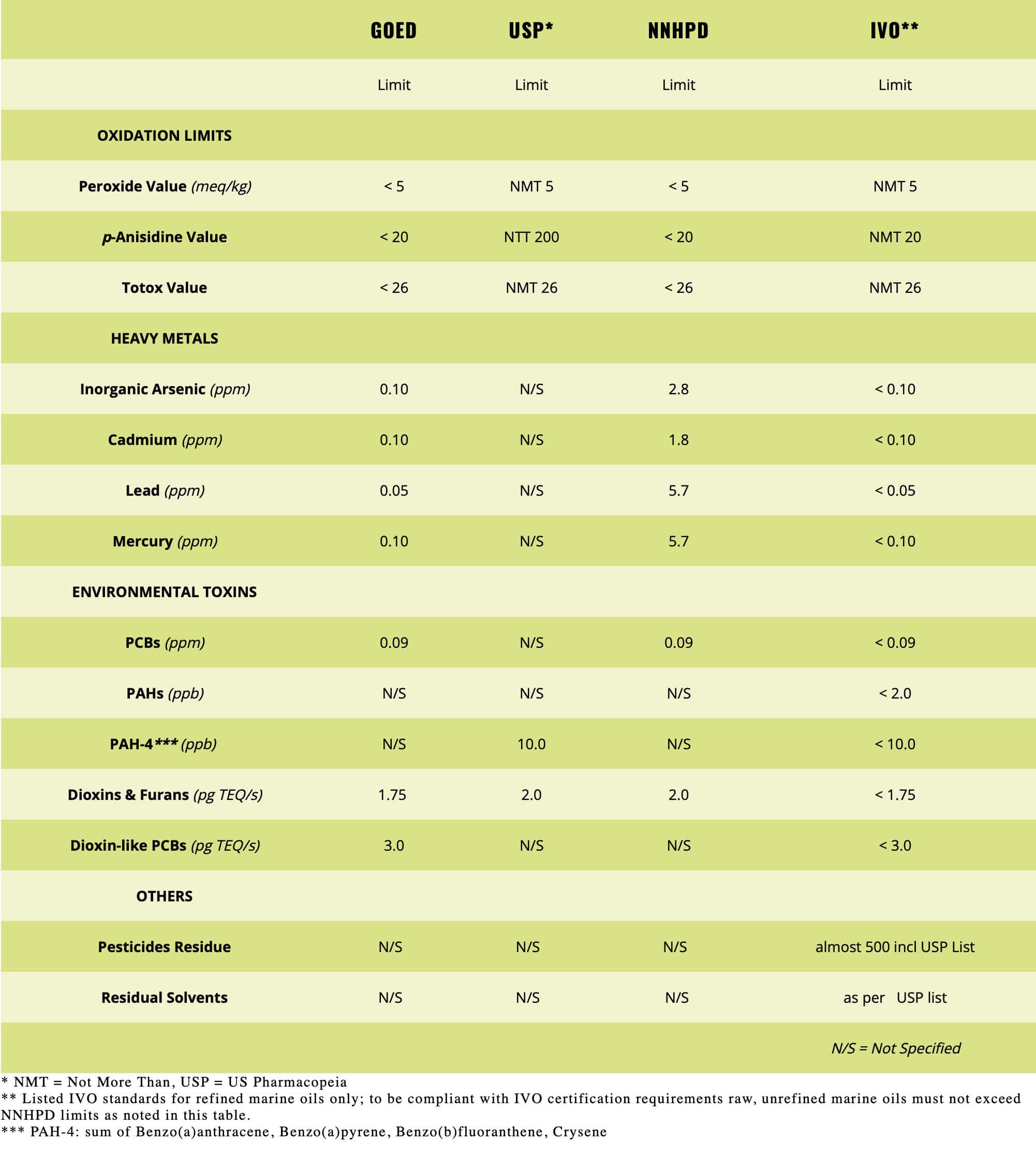
At its heart, IVO™ certification sets a new standard for quality and safety for edible marine-sourced oil testing because it requires a comprehensive set of quality checks including tests for some 500+ potential contaminants. . . from stem to stern, so to speak. The IVO seal is also your guarantee that the omega-3 oils meet all relevant international standards for purity, potency and sustainability, which includes the U.S. and Canadian regulations for foods and pharmaceuticals.
Today’s reality is such that our oceans are not as pristine as they once were. Therefore, once omega-3 rich fish are harvested, even from the cleanest waters, IVO certification requires fisheries and manufacturers to test for and subsequently purify the oils to reduce the already low levels of environmental contaminants. Every batch of omega-3 oil used in IVO certified products is tested for environmental toxins, including heavy metals, dioxins, PCBs, and other contaminants. These tests are conducted at the molecular level in parts per million and parts per billion. Mass spectrometry and other test methods are used to reach this high level of purity. The result is that the oils are more pure and contaminant-free than the fish itself!
To protect you and your family, the IVO seal is also proof that the potency or levels of omega-3 in the bottle meets or exceeds the guidelines established by regulating bodies or government agencies, such as:
- CFIA (Canadian Food Inspection Agency)
- CRN (Council for Responsible Nutrition)
- EPA (Environmental Protection Agency)
- EU (European Union)
- FDA (United States Food and Drug Administration)
- GOED (Global Organization of Omega-3 EPA and DHA Omega-3
- GRAS (Generally Recognized As Safe)
- HC (Health Canada)
- NFSA (Norwegian Food Safety Authority)
- NNHPD (Natural and Non-prescription Health Products Directorate)
- WHO (World Health Organization)
Mass Spectrometry Testing
Mass spectrometry provides the highest level of credibility and certainty of any analytical approach. IVO certified products must be submitted to state-of-the-art mass spectrometer testing that is capable of identifying pesticides, herbicides, fungicides, solvent residues and toxic heavy metals at ultratrace concentration levels.
Furthermore, all third-party test facilities must meet Good Laboratory Practice standards for analytical laboratories.
Here are some of the specialized mass spectrometry tests that are required of IVO certified products:
Headspace Gas Chromatography Mass Spectrometer
This instrument is used to detect volatile contaminants such as solvents.
- Able to detect residual solvents as low as 0.1 ppm (part per million), which is even lower than the specification of USP (United States Pharmacopoeia)
- Unknown compounds are easily identified by their mass spectral fingerprint through a library created by the NIST (National Institute of Standards and Technology)
Gas Chromatography Triple-Quadrupole Mass Spectrometer
This test instrument is used to detect semi-volatile and non-polar contaminants including:
- Many insecticides, fungicides, and herbicides
- Detects contaminants at parts per billion (ppb) levels
- More than 140 contaminants analyzed including:
- Organochlorine
- Organophosphorus
- Bridged diphenyl
- Pyrethroid insecticides
- Pyrethroid synergists
- Chlorinated aromatic fungicides
- Polycyclic aromatic hydrocarbons (PAHs)
- Dioxins, Dibenzofurans, Polychlorinated biphenyls (PCBs)
Inductively-Coupled Plasma Mass Spectrometer
The inductively-coupled plasma mass spectrometer tests for elemental impurities.
- Screens, identifies, and quantifies trace levels of heavy metal contaminants
- Screens for high levels of radioactive isotopes
- More than 100 times more sensitive than ICP technologies without mass spectrometry.
- Parts per billion level detection to ensure conformance with California’s Prop 65 limits on toxic elements.
Ultra High Performance Liquid Chromatography Triple-Quadrupole Mass Spectrometer
The ultra high performance liquid chromatography triple-quadrupole mass spectrometer tests for polar contaminants, including many pesticides and toxins.
- Ultra-selective, ultra-sensitive detection for water-soluble contaminants at trace levels
- Analyzes more than 100 contaminants including:
- Newer classes of pesticides
- Mycotoxins
- Veterinary drugs and antibiotics
- Plasticizers, such as phthalates
Testing for Radiation Contamination
IVO requires radioactive testing be conducted on marine oils from specific geographic areas identified as “at risk” due to the small but potential risk of radiation contamination in omega-3 oils from the source fish and/or krill. IVO certification sets a maximum radioactive tolerance of 100 Bq/kg which is far below the 300 Bq/kg limit mandated by Health Canada’s Natural and Non-prescription Health Products Directorate (NNHPD) for substances in which naturally occurring radioactive materials may be present.
To secure the most accurate results on radiation contamination, IVO works with the Saskatchewan Research Council (SRC), one of Canada’s leading providers of applied research, development and demonstration (RD&D) and technology commercialization. Samples are sent to SRC where they conduct a Gamma spectrometry measurement test. This test captures the occurrence and frequency of radioactive nuclides’ breakdown in the samples of omega-3 oils.
Note: 1 Bq/kg (Becquerel) is defined as the activity of a quantity of radioactive material in which one nucleus decays per second per kilogram of a substance. It is a standard measurement of radioactivity used for foods and soils.